Monitoring pH and chloride in concrete
Responsible: Yurena Seguí Femenias
Funding: Swiss National Science Foundation (SNSF)
Collaborations: Lafarge-Holcim Research Centre (Dr. Fabrizio Moro)
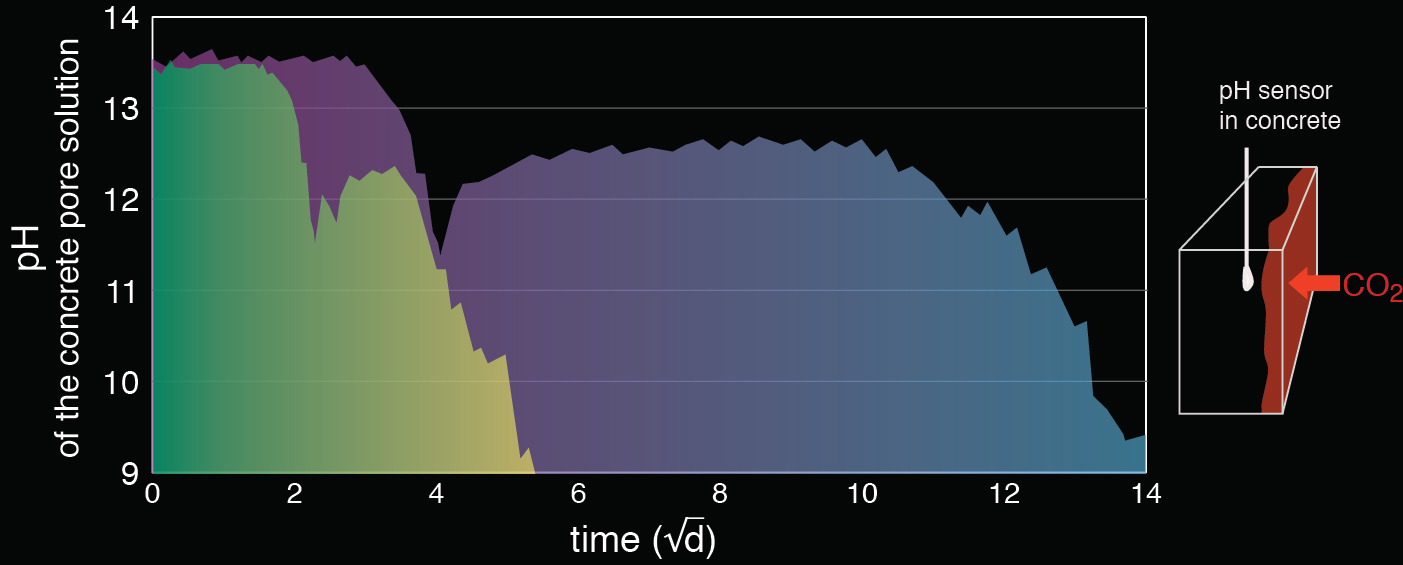

Background
For corrosion of steel in porous media such as concrete, both the pH and the concentration of chloride in the aqueous phase are crucial parameters. The non-destructive, continuous measurement of these two parameters is, however, a major challenge. This is true in laboratory studies and particularly on-site.
Aims and objectives
The aim of this project was to take advantage of recent developments made in this group with respect to potentiometric measurements of chloride concentrations and pH (see Refs. listed below) and to apply this technology for material characterization (durability performance of various cement types) and corrosion studies.
Methodology
Potentiometric sensors are based on measuring the sensor potential. In porous media such as concrete, however, different disturbing phenomena such as diffusion potentials arise and are known to have a major impact on the potential measurement (major potential error source). For potentiometric sensors to work properly, these error sources have thus to be taken into account carefully. We developed iterative algorithm (PhD thesis Y. Femenias, ETH Zurich, 2017) that considers these effects and allows to reliable determine pH and chloride concentrations in concrete. The potentiometric pH sensor is currently the only technology that allows the continuous, non-destructive measurement of pH over a wide range, including highly alkaline pH values (pH>13).
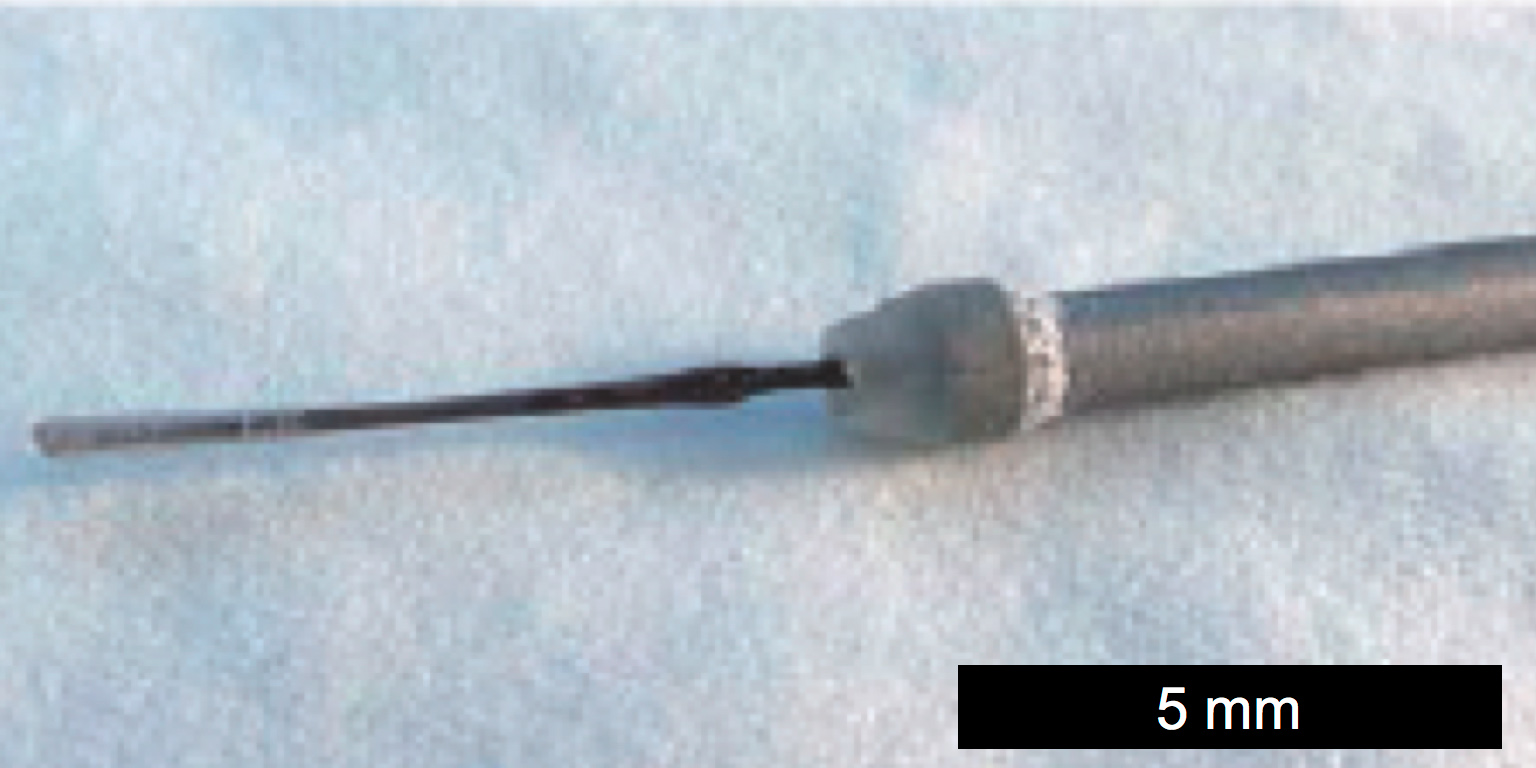
The picture below shows an example of a potentiometric chloride sensor (silver/silver chloride electrode).
References
- Angst U, Vennesland Ø. Detecting critical chloride content in concrete using embedded ion selective electrodes – effect of liquid junction and membrane potentials.
Materials and Corrosion 60 (2009) 638–643. external page doi:10.1002/maco.200905280 - Angst U, Vennesland Ø, Myrdal R. Diffusion potentials as source of error in electrochemical measurements in concrete.
Materials and Structures 42 (2009) 365–375. external page doi:10.1617/s11527-008-9387-5 - Angst U, Elsener B, Larsen CK, Vennesland Ø. Potentiometric determination of the chloride ion activity in cement based materials.
Journal of Applied Electrochemistry 40 (2010) 561–573. external page doi:10.1007/s10800-009-0029-6 - Angst U, Elsener B, Myrdal R, Vennesland Ø. Diffusion potentials in porous mortar in a moisture state below saturation.
Electrochimica Acta 55 (2010) 8545–8555. external page doi:10.1016/j.electacta.2010.07.085 - Angst U, Elsener B, Larsen CK, Vennesland Ø. Chloride induced reinforcement corrosion: Electrochemical monitoring of initiation stage and chloride threshold values.
Corrosion Science 53 (2011) 1451–1464. external page doi:10.1016/j.corsci.2011.01.025 - Angst U, Polder R. Spatial variability of chloride in concrete within homogeneously exposed areas.
Cement and Concrete Research 56 (2014) 40-51. external page doi:10.1016/j.cemconres.2013.10.010 - Femenias Y, Angst U, Caruso F, Elsener B. Ag/AgCl ion-selective electrodes in neutral and alkaline environments containing interfering ions.
Materials and Structures 49 (2016) 2637–2651. external page doi:10.1617/s11527-015-0673-8 - Femenias Y, Angst U, Elsener B. Monitoring pH in corrosion engineering by means of thermally-produced iridium oxide electrodes.
Materials and Corrosion (2017) 1–13. external page doi:10.1002/maco.201709715